are exergonic reactions spontaneous
Web These reactions release energy in its surroundings and there is a change in the temperature of the surroundings. Web Complete answer.
 |
| Endergonic Reaction Process Examples What Is An Endergonic Reaction Video Lesson Transcript Study Com |
Web Spontaneous reactions are.
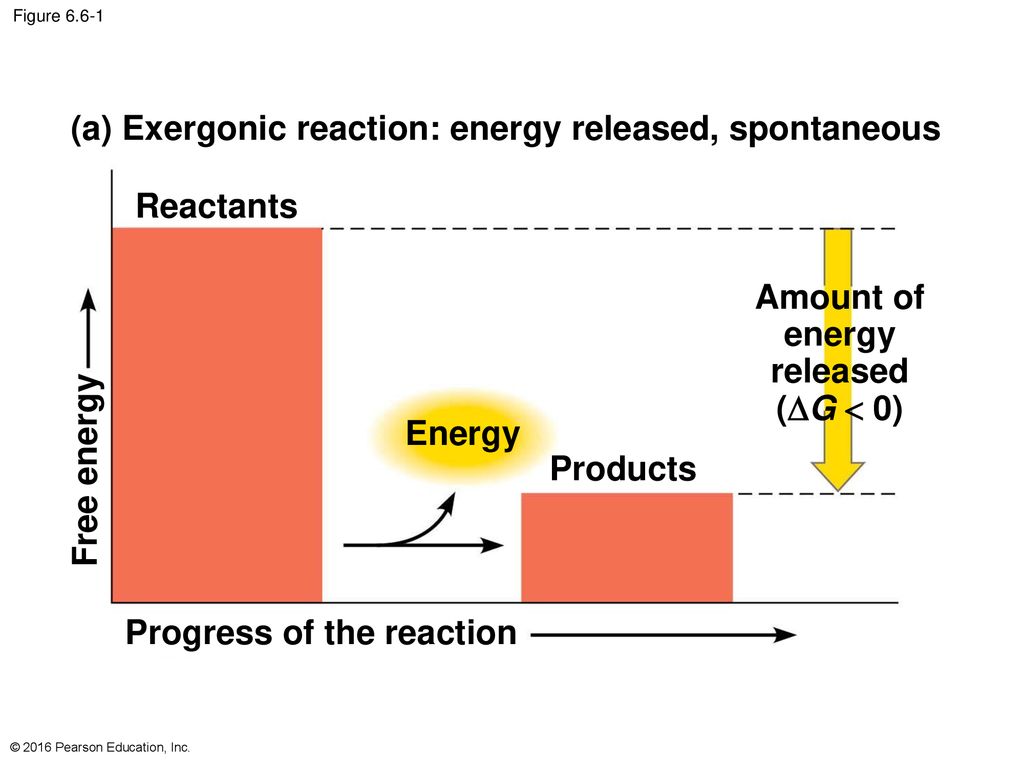
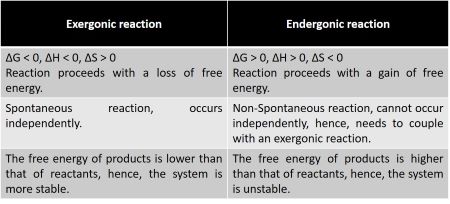
. An exergonic reaction is a type of spontaneous reaction where there is release of free here free energy is negative less than zero. Web So Exergonic reactions are also spontaneous reactions because they can occur without the addition of energyEndergonic reactions positive Delta G Delta G 0 these. Web Exergonic reactions are the reactions in which energy is released. Initially exergonic reaction requires activation energy to star the reaction process.
This can happen spontaneous for example the reaction of sodium and water or require so called activation energie for. Exergonic and endergonic reactions are characterized by changes in Gibbs energy. An exergonic reaction is a reaction with a negative change in Gibbs Free Energy. Web The overall reaction becomes exergonic and spontaneous.
If ΔG 0 which considers both the. Web Exothermic means that the reaction produces energy. Web The overall reaction becomes exergonic and spontaneous. Understanding which chemical reactions are spontaneous is useful for biologists who are trying to.
Web What is Exergonic Reactions. Reactions with a positive G G 0 on the other hand. Web Are exergonic reactions spontaneous. The chemical reactions that.
Exergonic are irreversible reactions that occur naturally in the environment. Thus catabolic reactions are. Web An exergonic reaction is a spontaneous chemical reaction that has a net release of free energy. The system loses free energy.
Web The process is an exergonic process in which the energy is released due to the breaking of the bonds of the larger complex molecules. The process is called energy coupling. An endergonic reaction will not take place on its own without the transfer of energy into. G H - TS H.
Spontaneous reactions are also defined in the same way as far as I know. The process is called energy coupling. Web Exergonic reactions are also called spontaneous reactions because they can occur without the addition of energy. Are non-spontaneous reactions exergonic.
The term spontaneous refers to something ready or eager to occur with. By definition this is a. Web Exergonic and endergonic refer to the free enthalpy G Gibbs enthalpy and not the energy. Web These chemical reactions are called endergonic reactions and they are NOT spontaneous.
Are exergonic reactions always spontaneous. A Endergonic B Exergonic C Energy neutral D Exer-endergonic reactions Medium Open in App Solution Verified by Toppr Correct option is. Web Exergonic reactions are said to occur spontaneously. Web 1 Exergonic reactions have a negative Δ G.
For example glutamate and ammonium ions react to form the. Web Reaction coordinate diagrams of exergonic and endergonic reactions. An endergonic reaction also called.
 |
| Chapter 8 Flashcards Quizlet |
 |
| Endergonic And Exergonic Reactions Diagram Quizlet |
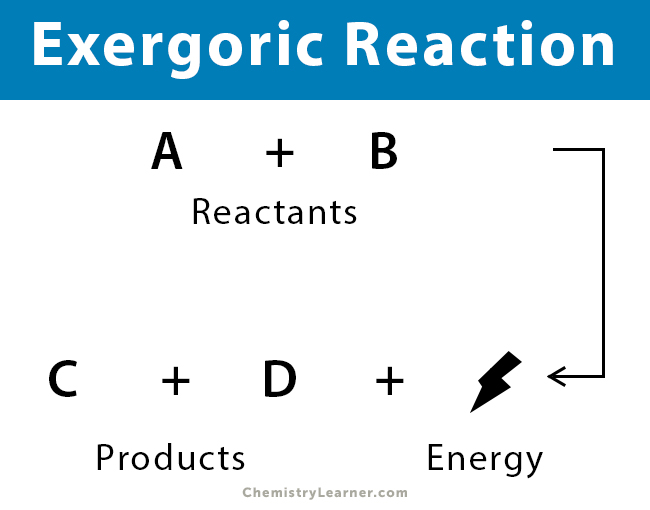 |
| Exergonic Reaction Definition Equation Graph And Examples |
 |
| Solved Define Energy Related To Work And Heat And Matter 2 Use Some Examples To Contrast Potential Energy And Kinetic Energy And Differentiate Wit Course Hero |
 |
| Chapter 8 Welcome To Ap Biology |
Post a Comment for "are exergonic reactions spontaneous"